Determination of the infectious nature of the agent of acute hepatopancreatic necrosis
Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp
Abstract
A new emerging disease in shrimp, first reported in 2009, was initially named early mortality syndrome (EMS). In 2011, a more descriptive name for the acute phase of the disease was proposed as acute hepatopancreatic necrosis syndrome (AHPNS). Affecting both Pacific white shrimp Penaeus vannamei and black tiger shrimp P. monodon, the disease has caused significant losses in Southeast Asian shrimp farms. AHPNS was first classified as idiopathic because no specific causative agent had been identified. However, in early 2013, the Aquaculture Pathology Laboratory at the University of Arizona was able to isolate the causative agent of AHPNS in pure culture. Immersion challenge tests were employed for infectivity studies, which induced 100% mortality with typical AHPNS pathology to experimental shrimp exposed to the pathogenic agent. Subsequent histological analyses showed that AHPNS lesions were experimentally induced in the laboratory and were identical to those found in AHPNS-infected shrimp samples collected from the endemic areas. Bacterial isolation from the experimentally infected shrimp enabled recovery of the same bacterial colony type found in field samples. In 3 separate immersion tests, using the recovered isolate from the AHPNS-positive shrimp, the same AHPNS pathology was reproduced in experimental shrimp with consistent results. Hence, AHPNS has a bacterial etiology and Koch's Postulates have been satisfied in laboratory challenge studies with the isolate, which has been identified as a member of the Vibrio harveyi clade, most closely related to V. parahemolyticus.
INTRODUCTION
The shrimp farming region of Southeast Asia andChina supports the largest and most productiveshrimp farming industry in the world. Beginning inabout 2009, a new, emerging disease called ‘earlymortality syndrome’ or ‘EMS’ (more descriptivelycalled acute hepatopancreatic necrosis syndrome orAHPNS; Lightner et al. 2012) began to cause signifi-cant production losses in southern China (NACA-FAO 2011). By 2010, the range of affected farms inChina had expanded, and by 2011, AHPNS was con-firmed in Vietnam and Malaysia (Lightner et al. 2012,Mooney 2012). EMS disease reached Thailand in2012 (Flegel 2012, Leaño & Mohan 2012). As hasbeen the case with other epizootic shrimp diseases(Stentiford et al. 2012), EMS is causing serious pro-duction losses in affected areas and is also impactingemployment, social welfare, and international mar-kets (Bondad-Reantaso et al. 2012, Mooney 2012).
Since its first emergence, the etiology of AHPNSremained unknown. Initial laboratory studies failedto demonstrate an infectious etiology. The pathologyof AHPNS is limited to the hepatopancreas (HP), andthe nature of that pathology suggested that the dis-ease had a toxin-mediated etiology (Lightner et al.2012). The gross signs of AHPNS are evident bypond-side examination of affected shrimp accompa-nied by dissection and examination of the HP. Thesesigns may become apparent as early as 10 d post-stocking in a recently prepared pond. Shrimp withearly AHPNS will show a pale to white HP due topigment loss in the HP R-cells, as well as atrophy ofthe HP that may reduce the expected size of theorgan by 50% or more. In the terminal phase of thedisease, black streaks or spots (due to melanin depo-sition from hemocyte activity) appear in the HP.
Following routine histological methods (Lightner1996), the histopathology of AHPNS presents as anacute progressive degeneration of the HP fromproximal to distal with dysfunction of R-, B-, F-,and E-cells. Affected HP tubule epithelial cellsoften display prominent karyomegaly. Such cellsround-up and detach from the affected HP tubules,and become necrotic within the HP tubules or inthe gut lumen. In the terminal phase of AHPNS,the HP shows marked intra- and inter-tubularhemocytic infiltration and development of massivesecondary bacterial infections that occur in associa-tion with the necrotic and sloughed HP tubuleepithelial cells. Lesions found in infected individu-als of both Pacific white shrimp Penaeus vannameiand black tiger shrimp P. monodon are identical(Lightner et al. 2012).
Preliminary studies to determine the causeof AHPNS
Two approaches were initially attempted by theAquaculture Pathology Laboratory at the Universityof Arizona (UAZ-APL) to determine the etiology ofAHPNS. These included studies aimed at: (1) findingan environmental toxin as the possible cause of thedisease and (2) testing for infectious agent(s).Included among the studies in (1) (environmentaltoxins) were water and sediment samples, algae fromAHPNS-affected ponds, feed samples (because of thepotential for mycotoxin) from affected farms, andpesticides used for killing white-spot syndrome virusvectors. None of these studies was successful atreproducing the pathology characteristic of AHPNS(Lightner et al. 2012).
Under point (2) (testing for an infectious agent),several infectivity studies were conducted at UAZ-APL using frozen materials collected from affectedfarms in Vietnam in 2011 and 2012. A number ofinfectivity methods were employed in an effort toreproduce AHPNS lesions in experimentally chal-lenged shrimp. The methods included: intramuscular(IM) injections with 0.45 µm filtered and unfilteredinocula and per os (feeding experimental shrimpwith AHPNS-infected shrimp carcasses). None ofthese methods was found to induce pathology of theHP consistent with AHPNS (Lightner et al. 2012).
Infectivity studies conducted in Vietnam
Because AHPNS was suspected to be caused by abiological agent, UAZ-APL had concerns that thepotential infectious agent in frozen materials mighthave been inactivated by freezing and thawing.Another concern was that the infection models, byIM injection conducted at UAZ-APL in preliminarystudies, might not be the most natural route of infec-tion for AHPNS. Based on those preliminary studies,the UAZ-APL conducted several on-site studies in anAHPNS endemic area in the Mekong Delta of Viet-nam during mid- to late 2012. Several challengemethods were used in which only live or fresh mate-rials (never frozen) from AHPNS-affected shrimpwere tested for pathogenicity. In July 2012, IM injec-tion with filtered and unfiltered AHPNS-infectedshrimp homogenates, reverse gavage (Aranguren etal. 2010), per os, and co-habitation studies were con-ducted. The results from these studies showed thatAHPNS lesions could be induced in experimentalshrimp in per os and co-habitation studies. Theseresults were confirmed by routine histological meth-ods as described by Bell & Lightner (1988) and Light-ner (1996).
Histological examination of infected shrimp consis-tently shows that the AHPNS pathology is limited tothe HP and that significant bacterial involvementdoes not appear within the HP during the acutephase of AHPNS. Based on the progression of thepathology, and the suggested nature of the infectionroute, as determined by the infectivity studies, weworked on the hypothesis that the causative agent(s)may colonize the shrimp digestive tract in the earlystage of infection and that those bacterial coloniesmight be able to produce a toxin(s) capable of caus-ing HP dysfunction.
In December 2012, another on-site study wasconducted in the Mekong Delta of Vietnam with the intent to focus on bacterial populations found inthe stomach and HP of infected shrimp collected inVietnam and not frozen. Two trials with a mixtureof bacteria isolated from the stomach and HP ofAHPNS- infected shrimp were run. With each mixedculture of bacteria, 2 challenge studies were con-ducted using either bacteria growing on solidmedia (tryptic soy agar with added 2% sodiumchloride, TSA+) fed to experimental shrimp, or bac-teria grown in liquid media (tryptic soy broth withadded 2% sodium chloride, TSB+) for the immer-sion study. Of these treatments, only the immersiontreatment with mixed stomach bacteria was foundto induce AHPNS-typical lesions, which were con-firmed by histological examination (unpubl. data).Based on the result that the mixed culture ofinfected shrimp stomach bacteria could induceAHPNS pathology, the same mixed culture wasbrought back from Vietnam for further studies con-ducted at UAZ-APL.
MATERIALS AND METHODS
Bacterial isolates
In Vietnam in December of 2012, the stomachsfrom AHPNS-positive shrimp were asepticallyremoved, minced, and separately inoculated intoflasks containing 30 ml of TSB+; the flasks werethen incubated at 28°C for 18 h to obtain mixedcultures. The mixed bacterial cultures found toinduce AHPNS by immersion experiments in Viet-nam were preserved in TSB+ with 25% addedglycerol, frozen in dry-ice, and stored at −20°Cprior to being transported frozen for experiments atUAZ-APL. Once at UAZ-APL, the mixed cultures ofinterest were subjected to sub-culture to obtainindividual colonies and pure cultures on TSA+plates. The only mixed-bacteria culture used in theexperiments at UAZ-APL was designated as mixedculture A, and 3 sub-cultures of mixed culture A,designated as A/1, A/2, and A/3, were also testedfor their pathogenicity in the experiments. Afterpure cultures were obtained, bacterial identifica-tions were conducted by using API Rapid NE testand 16S rRNA sequencing.
Media types
Both solid and liquid media were used for bacterialculture. The solid media used were TSA+, MarineAgarTM(Difco), and TSA+ with added 5% sheepblood (Blood Agar) in an attempt to isolate thecausative agent of AHPNS. The liquid medium usedto replicate the results from Vietnam was TSB+.
Immersion challenge tests
Both liquid and solid media were employed togrow the bacterial samples (both mixed and purebacterial cultures) for inoculum preparation forchallenge tests. For liquid media, the inocula wereprepared by separately inoculating the glycerol-preserved bacterial isolates into flasks containing30 ml of sterile TSB+, and then the flasks wereplaced in the rotary shaker and incubated for 18 hat 28°C. After 18 h of incu bation, TSB+ solutionswere checked using a spectrophotometer at OD600to determine bacterial den sity. Inocula grown onsolid media were prepared as follows: the glycerolpreserved isolates were inoculated in TSB+ for 4 hat 28°C in the rotary shaker before plating onto 3different solid media (TSA+, marine agar, andsheep blood agar) and incubating for 18 h at 28°C.Bacteria grown on solid media were scraped, andthen re-suspended in saline water for immersionexperiments. The immersion procedure was carriedout by immersing 15 shrimp for 15 min with aera-tion in a flask containing a solution of approxi-mately 150 ml prepared by mixing of either TSB+broth culture or the bacterial suspension obtainedfrom bacteria grown on solid agars with salinewater to achieve a bacterial density of approxi-mately 2 × 108cells ml−1. Following the 15 minimmersion in the bacterial suspension, this samesuspension was added directly into an experimentaltank containing clean artificial seawater to obtainan approximate bacterial density of 2 × 106cellsml−1, and then the immersed animals were trans-ferred into the experimental tank. Shrimp inthe negative control group were immersed insterile TSB+.
Reverse gavage challenge test
The reverse gavage challenge tests were conduc -ted following the method described by Arangurenet al. (2010). The broth medium, after being ino -culated with bacteria and incubated for 18 h, wascentrifuged at 6000 rpm (3200 × g) (5 min). The super-natant fluid was filtered through a 0.2 µm filter. Food colorant was added to filtered supernatant fluid before being delivered to the shrimp. Each experi-mental shrimp in the reverse gavage treatmentreceived 2 doses of 0.1 ml of the filtered supernatantthrough the anal route on Days 0 and 2. The negativecontrol shrimp received 2 doses of 0.1 ml of sterileTSB+ via reverse gavage on Days 0 and 2 of theexperiment.
Indicator animals
Animals used in the experiments were Kona linespecific-pathogen free (SPF) Pacific white shrimpPenaeus vannamei (Lightner et al. 2009, Moss et al.2012). The experimental shrimp ranged in weightfrom 0.5 to 2 g.
Tank preparation
Depending on the experimental design, either 90 ltanks or small 4 l glass jars were used. Each tank orjar was equipped with a submerged biological filterand filled with artificial seawater at a salinity of25 ppt. Water temperature was maintained at around26 to 28°C, dissolved oxygen was maintained above5 ppm, and total ammonia concentration was keptbelow 0.1 ppm. Experimental tank set-up followedthe methods described by White et al. (2002).
Expt 1: Immersion study with mixed bacteriaisolated from AHPNS-positive shrimp
Four 90 l plastic tanks were employed for 3 replica-tions of the immersion treatment with the mixed bac-teria isolated from infected shrimp stomach (mixedculture A) that previously had been shown to induceAHPNS in shrimp by immersion challenge tests con-ducted in Vietnam. One negative control tank wasincluded. Each tank was stocked with 15 SPFPenaeus vannamei.
Expt 2: Immersion challenge study with individualbacterial isolates
Three pure cultures (A/1, A/2, and A/3), isolatedfrom mixed culture A, were tested for pathogenicity.All 3 pure cultures were grown in TSB+ for inoculumpreparation for immersion challenge tests. The posi-tive control group was treated with the known, con-sistently pathogenic, mixed bacterial culture A, alsogrown in liquid media (TSB+). Sterile TSB+ was usedfor negative control in the challenge. In addition,mixed culture A was also grown on 3 different solidagars (TSA+, marine agar, and sheep blood agar), foran additional immersion challenge test. This experi-ment was carried out in 4 l jars containing 5 experi-mental shrimp each. Three replications were used foreach treatment.
Expt 3: Immersion bioassay with bacteria isolatedfrom AHPNS-positive treatments from Expt 2
The results obtained from Expt 2 indicated thatonly the A/3 colony from the shrimp stomach couldcause AHPNS pathology in SPF shrimp. In addition,the same type of colony was also recovered from thewater from the AHPNS-positive tank in whichshrimp were treated with mixed culture A. This iso-late was named B/1. The purpose of Expt 3 was torepeat the immersion experiment with the pure cul-ture of bacteria re-isolated from the infected shrimpstomach from Expt 2 in order to prove that AHPNS isa true infectious disease (Lightner 1988, Hasson et al.1995), and to complete Koch’s Postulates (Lightner1988, Saulnier et al. 2000, Hasson et al. 2009). Inaddition, because the results of Expt 2 indicated thatAHPNS could not be introduced by immersion treat-ments with bacteria grown on solid agars, a reversegavage study was conducted using the filtered super-natant fluid from TSB+ that was inoculated with A/3.An immersion treatment with the known pathogenicisolate, the mixed culture A, served as a positive con-trol. Negative control shrimp were treated with ster-ile TSB+ via reverse gavage. This experiment wascarried out in 4 l jars containing 5 experimentalshrimp each. Three replications were applied foreach treatment.
Observation and sampling
Shrimp were fed twice daily with a pelletedshrimp feed (35% protein, Rangen) for 5 d. Duringthe bio assay period, shrimp were checked approxi-mately every 6 h. Dead shrimp were removed fromthe experimental tanks and frozen at −70°C. Whenobserved, 1 to 2 moribund shrimp were fixed inDavidson’s AFA fixative for histology (Bell & Light-ner 1988, Lightner 1996). At termination (5 d), 2 to3 shrimp from each tank were fixed for histologywhile some were used for re-isolation of bacterialcultures.
Histopathology
All shrimp sampled for histopathology purposes were injected with AFA Davidson’s fixative, processed, and stained with hematoxylin and eosinphloxine (H&E) using routine histological methods described by Lightner (1996). The histological sections were analyzed by light microscopy for AHPNS/EMS lesions in the HP. Lesion severity was graded accordingly to the G-grading system (Lightner 1996), from G0 for negative to G4 as the highest severity of AHPNS/EMS.
RESULTS
Expt 1: Immersion study with mixed bacteria isolated from AHPNS-positive shrimp
Shrimp immersed in the bacterial broth containing mixed culture A began to develop AHPNS gross signs, and the first mortality occurred within 18 h. Mass mortalities occurred from 18 h postchallenge until Day 4, when the experiment was terminated because cumulative mortalities had reached 100% (Fig. 1). Gross signs presented by the challenged Penaeus vannamei included an empty gastrointestinal tract, whitish, ‘milky’ appearance of the stomach, whitish, atrophied HP, lethargy, and soft shells (Fig. 2A,B).

Fig. 1. Penaeus vannamei. Mortalities induced in the immersion bioassay using the mixed bacterial culture
Upon routine histological examination (Lightner 1996) using the G-grading system, negative control shrimp HPs appeared normal with normal structure of tubules and epithelial cells (Fig. 3A,B). In contrast, after 48 h, the HPs of shrimp treated with the mixed bacteria culture showed G2−3 HP tubule cell sloughing, G0−1 B-cells, virtually no R-cells, G0−1 F-cells, some development of nuclear karyomegaly of the HP tubule cells, some hemocytic infiltrates, and virtually no bacterial colonization. This was scored as an overall G3−4 AHPNS (Fig. 3C,F). After 72 h of exposure to the agent, AHPNS-infected shrimp HPs exhibited G0 lipids, G0 B-, F-, and R-cells, G3−4 sloughing, G3−4 hemocytic infiltration and bacterial colonization, and an overall G4 AHPNS/EMS (Fig. 3D,E).

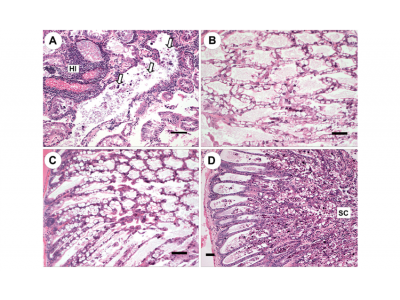
Fig. 2. Penaeus vannamei. (A,B) Gross signs of AHPNS-infected shrimp. Pale, atrophied hepatopancreas (HP), and an empty stomach (ST) and midgut (MG), which was induced by immersion bioassay. (C,D) Normal shrimp in the negative control group, showing a normal size HP with dark orange color and a full stomach and midgut. (B) and (D) are dissected individuals from (A) and (C), respectively
The result from Expt 1 indicated that the mixedbacterial culture isolated from AHPNS-infectedshrimp from Vietnam induced the same AHPNSpathology as described by Lightner et al. (2012) inSPF shrimp by immersion challenge. The results of Expt 1 reinforced the results of the immersion infec-tivity study conducted in Vietnam (UAZ-APL, unpubl.data) showing that the mixed bacterial culture fromthe stomach of infected shrimp induced AHPNSpathology during the immersion challenge study.

Fig. 3. Penaeus vannamei. Histological sections from the immersion bioassay. (A,B) Normal shrimp from the negative control group showing normal structure. (C−F) Lesions induced by immersion treatments with mixed culture A showing: (C) acute phase with no B-, F-, and R-cells, and sloughing of hepatopancreas (HP) tubule epithelial cells; (D) early terminal phase: necrotic sloughing of HP tubule epithelial cells (NC) and the remnants of HP tubules which are surrounded by hemocytic infiltration ; (E) terminal phase: sloughing cells (arrows) are shown in the HP tubule lumens along with significant bacterial colonization (BC), and remnants of HP tubules are surrounded by hemocytic infiltrates; and (F) affected tubules with no B-, F-, and R-cells, and some nuclei (arrows) of HP tubule epithelial cells are enlarged (karyomegaly). Scale bars = (A,B,D) 100 µm, (C,E) 50 µm, (F) 30 µm
Expt 2: Immersion challenge studies with individ-ual bacterial isolates
The resulting AHPNS typical gross signs and mor-talities presented by the challenged Penaeus vannamei indicated that the type of media used mayaffect the ability of mixed culture to induce thepathology in experimental shrimp (Table 1, Expt 2).Although the bacterial densities in all immersiontreatments were adjusted to 2 × 108cells ml−1, the different media types used to grow the bacteria gavedifferent results. The immersion treatment withmixed culture A grown in liquid media (TSB+)caused typical AHPNS gross signs and mortalities inexperimental shrimp within 18 h after exposure.Upon histological examination of fixed samples,100% of the samples in the TSB+ treatment werepositive for AHPNS pathology at high severity grades(G3−4). In contrast, all treatments with the samemixed culture A grown on solid media did not induceany AHPNS gross signs until 3 d after exposure, andno mortalities were recorded. Only one shrimpexposed to bacteria grown on marine agar showedsome low grade AHPNS gross signs. The histologicalexamination results of the other 2 treatments (TSA+and sheep blood agar) were negative for AHPNS.Regarding the treatments with the individual cul-tures A/1, A/2, and A/3 grown in liquid media(TSB+), the results clearly indicated that only onetype of colony from the mixed culture could replicatethe AHPNS pathology in experimental shrimp(Fig. 4D). Only shrimp in the treatment with bacterialisolate A/3 presented gross signs of AHPNS and mor-talities. In contrast to this result, the Penaeus van-namei in the 2 other treatments (A/1 and A/2)showed no signs of AHPNS by subsequent histologi-cal examination (Fig. 4B,C). Meanwhile, shrimp inthe A/3 group were positive for AHPNS at highseverity grades (Fig. 4D). Shrimp in the A/3 groupapproached 100% mortality on Day 2 of the chal-lenge test; in contrast, no mortalities were recordedin either the A/1 or A/2 groups (Fig. 5, Table 1).These results indicated that in the mixed culture A,there is probably only one type of bacteria capable ofinducing AHPNS pathology. The results also indi-cated that liquid media might be required for the sus-pected AHPNS-causing bacteria to be able to inducethe disease.
Expt 3: Immersion bioassay with bacteria isolatedfrom AHPNS-positive treatments of Expt 2
Expt 2 clearly indicated that only one bacterial iso-late (A/3) from mixed culture A could induceAHPNS. From shrimp challenged with isolate A/3,we re-isolated the same type of colony from anexperimentally infected shrimp’s stomach and re -peated the immersion experiment using the samemethod described above. Moreover, the same bacte-rial isolate was also recovered from the seawaterfrom the positive control tank of the previous immer-sion study (immersion with mixed culture A). Thisrecovered bacterial isolate was designated as B/1. InExpt 3, we tested both isolates (A/3 and B/1) recov ered from Expt 2 for pathogenicity. The positive control group was treated with the known, consistently pathogenic, mixed bacterial culture A that originated in Vietnam. All isolates were grown in liquid media (TSB+) for the challenge study. One additional treatment was also designed to test for toxicity of liquid media that had been inoculated with A/3, but had the bacterial cells removed by filtration using 0.2 µm filters. The reverse gavage technique (Aranguren et al. 2010) was applied to challenge SPF Penaeus vannamei in this treatment.
| Treatment | Media used | Number of shrimp | Cumulative mortality (%) | AHPNS histology |
| Expt 1 | ||||
| Mixed culture A | TSB+ | 45 | 100 | AHPNS positive G3−4 |
| Negative control | Sterile TSB+ | 15 | 0 | Negative |
| Expt 2 | ||||
| Mixed culture A (positive control) | TSB+ | 15 | 100 | AHPNS positive G3−4 |
| Pure culture A/1 | TSB+ | 15 | 0 | Negative |
| Pure culture A/2 | TSB+ | 15 | 0 | Negative |
| Pure culture A/3 | TSB+ | 15 | 100 | AHPNS positive G3−4 |
| Mixed culture A (TSA+) | TSA+ | 15 | 0 | Negative |
| Mixed culture A (marine agar) | Marine agar | 15 | 0 | Suspected AHPNS |
| Mixed culture A (blood agar) | Sheep blood agar | 15 | 0 | Negative |
| Negative control | Sterile TSB+ | 15 | 0 | Negative |
| Expt 3 | ||||
| Mixed culture A (positive control) | TSB+ | 15 | 100 | AHPNS positive G3−4 |
| Pure culture A/3 | TSB+ | 15 | 100 | AHPNS positive G3−4 |
| Pure culture B/1 | TSB+ | 15 | 100 | AHPNS positive G3−4 |
| Reverse gavage A/3 | Filtered TSB+ | 15 | 100 | AHPNS positive G3−4 |
| Negative control | Sterile TSB+ | 15 | 0 | Negative |
Table 1. Penaeus vannamei. Summary of immersion Expts 1, 2, and 3. AHPNS: acute hepatopancreatic necrosis syndrome;TSA+ (TSB+): tryptic soy agar (broth) with added 2% sodium chloride. Samples considered positive for AHPNS were evaluated using the G-grading system, ranging from G0 for negative to G4 indicating the highest severity

Fig. 4. Penaeus vannamei. Histological sections from the immersion bioassay showing the AHPNS lesions induced by pathogenic bacterial isolates. (A) Affected hepatopancreas (HP) of positive control (mixed culture A immersion), showing tubule epithelial cells sloughing (examples marked by arrows) and hemocytic infiltration (HI) surrounding remnants of necrotic HP tubules with sloughed HP cells in their lumens. (B,C) Normal HP tubule structure of A/1 and A/2 immersion. (D) Affected HP of shrimp in the A/3 immersion showing acute HP tubule degeneration and significant sloughing (SC) of HP tubule epithelial cells; the pathology progresses from proximal to distal. Scale bars = (A,B,C) 100 µm, (D) 50 µm

Fig. 5. Penaeus vannamei. Mortalities induced in the immersion bioassay using the mixed and pure bacterial cultures grown in liquid media
The results of this experiment confirmed that mixed culture A and A/3 caused AHPNS pathology both with typical AHPNS gross signs and histological changes (Fig. 6A,B). In addition, the recovered isolate B/1 from seawater of Expt 2 (which had an identical colony morphology as the A/3 isolate), also induced typical AHPNS pathology as was seen by both gross signs and histology (Fig. 6C). Interestingly, the reverse gavage treatment also caused AHPNS gross signs as well as histological changes at the microscopic level, characterized by acute HP cell sloughing and inter- and intra-tubular hemocytic infiltration (Fig. 6D). Shrimp treated on 2 consecutive days by reverse gavage with 0.2 µm-filtered supernatant from TSB+ (media was ino culated with A/3 and incubated for 18 h at 28°C) showed typical AHPNS pathology.
Fig. 6. Penaeus vannamei. Histological sections from the immersion and reverse gavage bioassays showing AHPNS lesions. (A) Mixed culture A immersion treatment. Arrows: examples of hepatopancreas (HP) tubule epithelial cells sloughing into the HP tubule lumens. Significant bacterial colonization (BC) can also be observed within of remnants of HP tubules. (B) Pure culture A/3 immersion treatment: no B-or R-cells are apparent. HP tubule epithelial cells are shown sloughing (SC) into the HP tubule lumens. (C) Pure culture B/1 immersion treatment. No B-or R-cells are apparent. Arrows: examples of acute cell sloughing of HP tubule epithelial cells. (D) Reverse gavage treatment: shown are necrotic and sloughed HP tubule epithelial cells (NC) and marked hemocytic infiltration among HP tubules. Scale bars = 50 µm
In general, experimental shrimp subjected to im - mersion treatment with pure cultures (A/1 and B/1) showed acute progression of AHPNS, characterized by rapid development of typical gross signs. Mortality in treatments with pure cultures of bacteria occurred after 18 h of exposure and approached 100% after 48 h of exposure. Mortality in the treatment with the mixed culture A was slower than with the treatments with pure cultures. Similarly, shrimp in the reverse gavage treatment tended to survive longer than those subjected to the bacterial immersion treatments (Fig. 7). However, the AHPNS histological lesions in reverse gavage treatments were very similar to those exhibited in shrimp from the treatments with immersion of bacterial suspensions (Fig. 6D). These results indicate that only one type ofbacterial colony is responsible for causing AHPNSpathology in laboratory conditions, and that a filter-able toxin is responsible for the observed pathologyto the HP.

Fig. 7. Penaeus vannamei. Mortalities induced in the immersion and reverse gavage bioassays using the mixed and pure bacterial cultures grown in liquid media
Bacterial identification
The only bacterial isolate (pure cultures A/3) fromthe mixed culture that showed pathogenicity in thisstudy was identified as Vibrio parahaemolyticususing API Rapid NE test kits and 16S rRNA sequen-cing. The detailed taxonomy of the bacterial speciesthat causes AHPNS will be reported in a subsequentpaper (L. Nunan et al. unpubl.).
DISCUSSION
Based on the nature of AHPNS pathology, a seriesof infectivity studies was designed to identify thesource and mode of infection. The on-site studies inVietnam took advantage of the location in anAHPNS-affected region to conduct several studiesusing fresh, never frozen, samples of Penaeus van-namei and P. monodon exhibiting gross signs ofAHPNS. Subsequent histological examination deter-mined the positive AHPNS status for the inocula thatwere prepared from locally obtained AHPNS-posi-tive shrimp. The on-site studies indicated thatAHPNS could not be transmitted through intramus-cular injection challenges, while per os and co-habi-tation studies confirmed that AHPNS was an infec-tious disease.
Histopathology studies indicated that in the earlyphase of the disease, the only affected organ is theHP. HP malfunction is characterized by tubuleepithelial cells sloughing into the HP tubule lumenswell before there is any indication of a causativeagent (e.g. causative bacteria). This was the impetusfor focusing on the stomachs of AHPNS-positiveshrimp as a probable source of the causative agent ortoxin of AHPNS, and efforts were made to cultureaseptically excised stomachs from affected Penaeusmonodon.
The initial infectivity studies conducted in Viet-nam, using a mixture of bacteria isolated fromAHPNS-positive stomachs and grown in liquidmedia, were found to produce AHPNS pathology inSPF shrimp via an immersion challenge test. Thisresult was a strong indication that AHPNS is causedby a cultivable, infectious agent that could be foundin stomachs of infected shrimp. Furthermore, thesestudies using the bacterial agent of AHPNS satisfiedthe 4 points of Koch’s Postulates (Lightner 1988,Saulnier et al. 2000, Hasson et al. 2009). The reasonswhy bacteria grown on solid media did not causeAHPHS were not determined. However, cell to cellsignaling in broth culture, as opposed to lack of spacefor this activity among bacteria grown on solidmedia, may be an area for future studies.
The results from this research also indicated thatthe bacteria-free supernatant of the broth mediacould induce AHPNS pathology in the reverse gav-age experiment. This evidence strongly suggests thatAHPNS lesions are caused by bacterial toxin(s), aswas suggested in a previous paper on AHPNSpathology (Lightner et al. 2012).
Future work will focus on characterizing the agentof AHPNS using traditional biochemical methods aswell as 16S rRNA sequencing (L. Nunan et al.unpubl.), developing diagnostic methods (possiblyusing PCR to detect the toxin-producing gene of theagent), and developing methods that may be usefulat managing AHPNS in affected countries.
Acknowledgements. We thank CP Foods, Bangkok, Thai-land; Uni-President Feed Company, Ho Chi Minh City, Viet-nam; Grobest Industrial (Vietnam) Company, Dong NaiProvince, Vietnam; Minh Phu Seafood Corp. Ho Chi MinhCity, Vietnam; Food and Agriculture Organization of theUnited Nations (FAO); the World Animal Health Organiza-tion (OIE); the World Bank; and the Responsible AquacultureFoundation of the Global Aquaculture Alliance for their sup-port of this research. We also thank P. Hoang, an undergrad-uate student at Nong Lam University at Ho Chi Minh City,for help in running the bioassays conducted in Vietnam.
Authors:
Loc Tran, Linda Nunan, Rita M. Redman, Leone L. Mohney, Carlos R. Pantoja, Kevin Fitzsimmons, Donald V. Lightner
Có thể bạn quan tâm
Phần mềm

Phối trộn thức ăn chăn nuôi

Pha dung dịch thủy canh

Định mức cho tôm ăn

Phối trộn phân bón NPK

Xác định tỷ lệ tôm sống

Chuyển đổi đơn vị phân bón

Xác định công suất sục khí

Chuyển đổi đơn vị tôm

Tính diện tích nhà kính

Tính thể tích ao hồ

 Early Mortality Syndrome (EMS) as new Emerging Threat…
Early Mortality Syndrome (EMS) as new Emerging Threat…  Microsporidian Impacts Shrimp Production – Industry Efforts Address…
Microsporidian Impacts Shrimp Production – Industry Efforts Address…