Measuring Dissolved Oxygen Concentration in Aquaculture
Dissolved oxygen concentration (DO) is considered the most impor-tant water quality variable in fish culture. In the broadest sense, how-ever, dissolved oxygen concentra-tion is no more important than other environmental variables because any factor that is outside the range tolerated by fish can cause stress or death. What makes dissolved oxygen concentration so important in intensive fish culture is the speed with which it can change. Over a matter of hours, or sometimes even minutes, DO can change from optimum to lethal lev-els. No other critical environmental variable in fish culture is so dynamic.
The dynamic nature of dissolved oxygen results from the interaction of three factors. First, oxygen is not very soluble in water so water has only a limited capacity to “hold” oxygen. Second, the rate of oxygen use by fish, plankton and organ-isms living in the pond mud can be high. Third, oxygen diffuses very slowly from the atmosphere into undisturbed water. The combina-tion of these three factors—limited solubility, rapid use and slow replenishment—can cause rapid changes in dissolved oxygen con-centrations.
Dissolved oxygen levels can be managed with aeration, but the response time for taking corrective measures is short. This makes it critical to have a rapid and reliable method of measuring dissolved oxygen concentrations so that aera-tion devices can be activated when needed.
There are a number of ways to measure dissolved oxygen concen-tration. Select a method based on 1) the number of ponds or tanks to be measured, 2) the level of accura-cy required, and 3) the cost of the measurement technique.
The titration-based “drop count” method fairly rapidly assesses whether or not there is sufficient dissolved oxygen in water. The drop count method is inexpensive and appropriate if DO concentra-tion is to be measured infrequently in a few ponds or tanks. However, on commercial fish farms or in any other situation where DO measure-ment of many ponds or culture units is routine, a dissolved oxygen meter is an indispensable piece of equipment.
What is an oxygen meter?
An oxygen meter has two compo-nents—the sensor (sometimes called the probe) and the meter. Various types of sensors are avail-able, but they all operate in basi-cally the same way: the sensor reacts with oxygen and an electri-cal signal is produced in propor-tion to the oxygen concentration.
The signal is then amplified, trans-lated into concentration units, and displayed by the meter. The meter circuitry also compensates the reading for changes in tempera-ture, altitude or salinity. The meter circuitry may also include features to aid in calibration.
Most DO sensors operate as elec-trochemical cells with a positive electrode (cathode) and a negative electrode (anode) connected by a “salt bridge” consisting of a satu-rated electrolyte solution. In most sensors, oxygen passes through a permeable membrane and is chemically reduced within the sensor. The chemical reduction of oxygen generates an electrical cur-rent that is processed by the elec-tronic components within the meter and displayed as a DO con-centration. The current is propor-tional to the concentration. Thus, DO meters do not measure oxygen concentration directly, but mea-sure a voltage that is produced by the chemical reactions of oxygen with the various components of the sensor.
Types of dissolved oxygen sensors
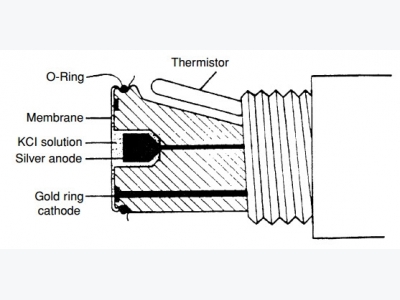
Polarographic or Clark sensors use gold or platinum as the cath-ode and silver as the anode (Fig. 1). Polarizing voltage is applied to the cathode to cause the reduction of oxygen within the sensor.
Oxygen is consumed at the cath-ode according to the reaction: O2 + 2H2O + 4e- 4OH-. In response to the production of hydroxyl ions (OH-) at the anode, and in order to preserve the charge balance of the electrolyte (saturated KCl) solu-tion, chloride ions react with silver at the anode according to the reac-tion: Ago + Cl- AgCl. Therefore, the chloride ions in the electrolyte solution function as a “carrier” of the electric potential.
Galvanic sensors use silver or platinum as the cathode and lead, iron or zinc as the anode. Application of a polarizing voltage is not necessary because the reduc-tion of oxygen in the presence of the sensor materials is sponta-neous. Thus, a galvanic sensor is like a battery (fuel cell) that is fueled by oxygen. Galvanic sensors typically have faster response times than polarographic sensors and are more expensive.
Fiber optic oxygen sensors consist of an optical fiber with a sensor tip that contains a thin layer of oxy-gen-sensitive fluorescent dye dis-solved in pure silicon. The optical fiber carries blue light from a light-emitting diode (LED) to the sensor. This stimulates the dye to emit flu-orescent light that travels back up the optical fiber to a photodetector. Oxygen diffusing into the sensor tip binds to the fluorescent dye, which reduces (“quenches”) the intensity of light emission. The extent of quenching is directly related to oxygen concentration. Fiber optic sensors are very sensi-tive at low DO concentrations. Fiber optic sensors are sensitive to ambient light, but this problem can be overcome by coating the sensor tip with silicon. However, this sili-con overcoat will reduce probe response time.

Figure 1. A cross section of a typical polarographic dissolved oxygen sensor.
Which oxygen meter is best?
Many different oxygen meters are commercially available (see list of manufacturers below), and each model has a unique combination of features that makes it more or less suitable for a particular applica-tion. The best meter for occasional use in an indoor setting, such as a hatchery, will be quite different from the one that is best for regular use under rough, outdoor condi-tions. The purchase decision is fur-ther complicated by the fact that good meter systems are expensive, primarily because precious metals are used in the construction of many sensors. Before buying a meter, consult with other fish farm-ers, Extension specialists, aquacul-ture supply companies, and meter manufacturers to identify the most suitable one.
Some of the desirable features of a dissolved oxygen meter suitable for making field measurements include:
■ accuracy
■ rapid response
■ ease of calibration
■ water resistance
■ sturdy, rugged construction
■ automatic temperature com-pensation
■ manual salinity compensa-tion
■ manual barometric pressure compensation
■ measures from 0 to 200 per-cent saturation
■ easily changed cable or probe
Other desirable features may include:
■ at least a 25-foot cable
■ a digital, liquid crystal dis-play that can be read in bright sunlight or in total darkness
■ an integral membrane cap assembly
■ a built-in calibration cham-ber/storage sleeve
■ storage of measured values in memory within the meter (datalogging)
■ an RS-232 personal computer interface
■ a “hold” or “auto-read” func-tion indicating that a stable reading has been attained
■ a battery charger
Operating an oxygen meter
It is beyond the scope of this publi-cation to provide detailed opera-tional instructions for each of the many kinds of meters. Carefully read the instructions that come with the meter to understand how it works and how to use it proper-ly. Remember, the number dis-played by the meter is not neces-sarily accurate. The number on the display is correct only when the meter has been properly calibrated, the measurement is made correctly, and the sensor and meter have been properly maintained. One common step in the use of most meters is calibration, and some details of this process are present-ed in the next section.
Calibrating an oxygen meter
Dissolved oxygen meters do not measure dissolved oxygen concen-tration directly, but measure an electrical current that is propor-tional to dissolved oxygen concen-tration. Therefore, the meter must be standardized to a reference con-dition that compares an electrical current to a known DO concentra-tion. The most common reference condition is the oxygen concentra-tion of air saturated with water vapor (100 percent humidity).
There are several techniques for calibrating meters. Consult the operations manual for specific instructions. Moist air calibration is the simplest and most straightfor-ward method. The following guidelines describe moist air cali-bration of dissolved oxygen meters with commonly used polarograph-ic sensors. Dissolved oxygen meters should be calibrated before each use.
(1) Inspect the sensor for bubbles under the membrane, and tears or fouling of the mem-brane.
Bubbles prevent proper meter calibration and cause readings to fluctuate erratically. Before placing the membrane on the sensor tip, lightly tap the probe body while holding the probe vertically upright to dislodge bubbles formed when filling the probe body with electrolyte solution. Replace the mem-brane if bubbles are present (Fig. 2). Wipe the membrane with a smooth cloth to remove water drops from the mem-brane surface.
(2) Place the sensor in a bottle with a moist sponge or paper towel in the bottom.
Calibration must occur in an environment in which the sur-rounding air is saturated with water vapor. Otherwise, cali-bration will be inaccurate and DO measurements will be erro-neous.
(3) Turn the instrument on.
If the instrument does not acti-vate, check the condition of the batteries.
(4) Zero the meter.
Adjust the display to read zero.
(5) Polarize the sensor in the cali-bration mode of the meter.
It takes about 15 minutes to completely consume the oxy-gen in the electrolyte solution behind the membrane and fully polarize a polarographic sen-sor. For the most accurate mea-surements, the sensor should be polarized and the meter cal-ibrated at a temperature as close to the pond water tem-perature as possible. Adjust the displayed value of dissolved oxygen to 100 percent satura-tion. If the displayed value slowly drifts lower, the sensor is not yet fully polarized.
Some DO meters must be cali-brated based on the saturation concentration of dissolved oxy-gen (in mg/L). Determining the DO concentration at saturation requires measuring air temper-ature and salinity and knowing the local altitude. Some DO meters have tables on the back of the meter or in the manual that show the saturation con-centrations of dissolved oxygen as a function of temperature and salinity.
(6) Switch the mode of the meter from calibration to that used to read dissolved oxygen con-centration.


Figure 2. Sequence for installing a membrane on a polarographic dissolved oxygen sensor.
A - Fill the sensor body with electrolyte solution.
B - Hold a membrane between the thumb of your left hand and the sensor body.
C - With your other hand, stretch the membrane up, over and down the other side of the sensor
body.
D - Secure the membrane to the sensor body under your forefinger.
E - Roll the O-ring over the sensor tip; pull out any wrinkles that may occur; check for trapped
bubbles.
F - Trim excess membrane from sensor tip.
(Figure courtesy of YSI Incorporated.)
Making dissolved oxygen measurements
The process of measuring dis-solved oxygen concentration with an oxygen meter is simple, although it involves a bit more than simply putting the sensor in the water and reading a number on the display. Be sure to read the instructions for the meter carefully as the procedure can vary some-what depending on the type of sensor being used.
Accurate measurement of DO con-centration with polarographic sen-sors requires moving the sensor in the water. Oxygen is consumed across the membrane, so failure to move the sensor will create an oxy-gen-depleted microzone around the sensor tip and show a measure-ment that is too low. Move the sen-sor up and down or side to side about 1 foot per second to prevent this problem. The response of dis-solved oxygen sensors is not instantaneous. Measured values will change rapidly at first and then begin to stabilize within 15 to 20 seconds, although it is rare that measured values in ponds will fully stabilize.
Dissolved oxygen measurements made near the pond bank are very different (usually lower) than those made in open areas of the pond because of the effects of sediment respiration and pond bank vegeta-tion. Some fish farmers attach the sensor cable to a pole, leaving about 1 to 2 feet of the cable and sensor to hang from one end of the pole, in order to measure farther from the bank (Fig. 3).
Other fish farmers tie the sensor to a small float (such as a seine float) so that the sensor dangles a foot or so beneath the float. The float and sensor are tossed from the pond bank out into the pond and then slowly retrieved while reading the meter. It is difficult to obtain an accurate measurement of DO con-centration using this method because it is usually not possible to pull the rig through the water long enough for the displayed value to stabilize. Nonetheless, this approach is preferable to measure-ments made near the pond bank and millions of pounds of fish have been successfully raised using this technique. The success of this approach is not based on accuracy but on the fact that this method always shows a DO con-centration that is too low. By sys-tematically erring on the low side, the pond can be aerated before dis-solved oxygen concentration becomes critical.
Typically, the DO concentration of a number of ponds or tanks is measured during a brief time. The dissolved oxygen meter should not be turned off between measure-ments to avoid depolarizing the probe. If the meter is not going to be used for about 1 hour, turn off the meter to extend the life of the batteries and the sensor. Recalibrate the meter when it is used again. If a large meter adjust-ment is necessary during recalibra-tion it may be an indication of problems with the membrane or sensor.

Figure 3. The cable connecting the dissolved oxygen meter to the probe can be attached to a pole to make measuring easier.
Dissolved oxygen sampling programs
When an oxygen meter is main-tained properly and used accord-ing to the manufacturer’s instruc-tions, extremely accurate measure-ments can be made. However, aquaculture systems are dynamic, and a single measurement of dis-solved oxygen, no matter how accurate, may have no practical significance because it represents just one of many possible values that can vary over time and from place to place, even in well-mixed, continuously aerated systems such as tanks or raceways. For example, feeding activity increases oxygen uptake by fish, so concentrations of dissolved oxygen decrease shortly after fish are fed. Also, pronounced gradients of dissolved oxygen con-centration often exist along race-ways because fish use oxygen as the water passes through each raceway section.
Using an oxygen meter correctly is often the easy part of implement-ing a good dissolved oxygen moni-toring program; the difficult part is determining when and how often to measure, where to measure, and how to interpret the measurement once it is made. Regrettably, there are no established rules for dis-solved oxygen monitoring pro-grams in aquaculture. The sam-pling protocol must be based on experience, the needs of the pro-gram, and good judgement. method always shows a DO con-centration that is too low. By sys-tematically erring on the low side, the pond can be aerated before dis-solved oxygen concentration becomes critical.
Compromises must often be made between obtaining accurate mea-surements and the logistical con-straints associated with sampling a large number of ponds in a timely manner. These compromises should be made with knowledge of the factors that affect DO con-centrations and the ways these fac-tors cause DO concentration to vary over time and from place to place.
Some of the factors to consider when monitoring DO concentra-tions in ponds (the most common use of oxygen meters in warm-water aquaculture) are discussed below.
Dissolved oxygen monitoring in ponds
Dissolved oxygen concentrations in ponds vary with depth, from one side of a pond to the other, and particularly from one time of day to another. In general, dis-solved oxygen concentrations are lowest at dawn and highest at dusk; lowest near the bottom and highest near the surface; and low-est near the upwind side and high-est near the downwind side.

Figure 4. The trend in the dissolved oxygen concentration in a fish pond over several days can help to predict the need for emergency aeration.
A dissolved oxygen measurement applies only to the conditions at the particular location of the mea-surement, and does not reflect sur-rounding conditions. A dissolved oxygen measurement also repre-sents a snapshot in time and therefore provides no information about the rates of processes that supply oxygen to the pond (photo-synthesis by plants, diffusion from the atmosphere) or the rates of processes that remove oxygen from the pond (respiration by fish, and organisms in the water and mud). The relative rates of these supply and removal processes vary with time and location in ponds and are responsible for the observed variation in dissolved oxygen concentrations. These phe-nomena and the way they affect the distribution of dissolved oxy-gen are discussed in any good publication on water quality in aquaculture ponds.
The more intensive the culture sys-tem the greater the need for rou-tine water quality monitoring. In ponds with few fish or crustaceans and low inputs of fertilizer or feed, biological activity is slow, even in summer, and phytoplankton blooms are light. Dissolved oxy-gen concentrations seldom, if ever, reach critically low levels and are relatively consistent with time and location in the pond. As the inten-sity of culture increases, biological activity increases and phytoplank-ton become more abundant.
During the summer months, DO concentrations may vary greatly from dawn to dusk, and concen-trations may change very quickly. On calm, sunny days, there may be substantial differences in dissolved oxygen concentrations from the surface of the pond to the bot-tom, even in relatively shallow ponds. Measuring a dissolved oxy-gen profile from the pond surface to the bottom will indicate the potential risk associated with the mixing, or turnover, of low-oxygen bottom water with high-oxygen surface water.
In high-density commercial ponds, at least two measurements should be made each day during the growing season. Normal daily extremes can be determined by measuring at dawn and at dusk. During the warm months, critically low dissolved oxygen concentra-tions usually occur at night, and it may be necessary to sample every hour or two to prevent problems.
On large commercial farms it is often impractical to sample from more than one location in each pond, so management decisions often must be made on the basis of a single sample. This is difficult because it is not possible to select a single point in the pond that repre-sents the average condition, or even the worst-case or best-case condition. Therefore, it is impor-tant to always sample each pond at the same location and at the same time of day to minimize variations. If the sampling program is consis-tent, the behavior of the fish can be correlated to dissolved oxygen con-centration, even if the actual con-centration where DO is measured has little to do with fish behavior in the pond.
Some consistency can be achieved by sampling every time from the same location in an individual pond and by sampling from the same relative position in all ponds. For example, one might sample from the south side of each pond just for consistency, or from the windward side of each pond to minimize differences caused by changes in wind direction. Pond surface DO concentrations do not reflect “average” or “worst-case” conditions. Therefore, measure-ments should be made at least 2 feet from the pond bank and 12 to 18 inches beneath the surface. The probe cable can be attached to a pole mounted on a vehicle. Of course, measurements should not be made near aeration devices.
A consistent measurement pro-gram allows the farmer to identify “problem” ponds, those in which aeration must begin earlier in the evening than in other ponds. Monitoring DO concentration in the same place at the same time each day also makes it possible to spot trends over several days so that the need for aeration in a par-ticular pond can be anticipated (Fig. 4). Therefore, obtaining an accurate dissolved oxygen mea-surement may be less important than assessing changes in DO con-centration over time and attempt-ing to link it with fish behavior (e.g., reduction in feed consumed, distress).
Caring for dissolved oxygen meters
Most dissolved oxygen meters designed for field use are rugged and can withstand the occasional rough treatment associated with normal use. Most are water-resis-tant, which means that they can tolerate rainfall, but are not water-proof, which means that they can not tolerate submergence. Water inside the meter will cause an erratic response when making a dissolved oxygen measurement. If a meter is accidentally submerged, open the case and place the meter in a warm, dry location.
A meter that is dropped or jostled excessively may need to be recali-brated. If the meter does not func-tion, have it professionally ser-viced.
Dissolved oxygen meters are con-nected to sensors by a cable. The point where the cable enters the sensor may be a weak point. The stress of repeated use can disrupt signal transmission from the sen-sor to the meter, which is seen as erratic meter response when the sensor is moved in the water
Stress on the junction between cable and sensor can be relieved by forming a loop with the cable near the probe and attaching the cable to the probe body with a cable tie or duct tape.
Changing membranes is probably the most important routine mainte-nance task that must be performed on dissolved oxygen meters. If there are tears or bubbles beneath the membrane it must be changed.
During heavy use (e.g., summer), the electrolyte solution should be changed monthly. Figure 2 illus-trates how to change a membrane on some polarographic sensors. With some practice and skill, a membrane can be changed in less than 2 minutes. Many polaro-graphic dissolved oxygen sensors now use an integral membrane with a plastic cap that screws onto the sensor body. However, integral membrane caps are much more expensive than the traditional membrane/O-ring combination.
The reactions that take place at the electrodes can cause coatings to form; these slow calibration or meter response time. In a polaro-graphic sensor, the gold cathode should be bright yellow and the silver anode should not be tar-nished or black. The cathode can be cleaned with emory paper, but the silver anode should be cleaned only by a qualified service repre-sentative.
Related news
Tools

Phối trộn thức ăn chăn nuôi

Pha dung dịch thủy canh

Định mức cho tôm ăn

Phối trộn phân bón NPK

Xác định tỷ lệ tôm sống

Chuyển đổi đơn vị phân bón

Xác định công suất sục khí

Chuyển đổi đơn vị tôm

Tính diện tích nhà kính

Tính thể tích ao



 Total Alkalinity, Total Hardness
Total Alkalinity, Total Hardness  Dissolved Oxygen Management In Aquaculture
Dissolved Oxygen Management In Aquaculture